A randomised, double-blinded, controlled trial to determine the efficacy of combined therapy of oclacitinib and marine oil extract PCSO-524 in dogs with atopic dermatitis
Summary
Seventeen client-owned dogs with Atopic Dermatitis were randomly assigned one of two treatments: Oclacitinib combined with PCSO-524, or the placebo of oclacitinib with sunflower oil. The researchers wanted to prove that the anti-inflammatory properties of PCSO-524 could complement the effect of oclacitinib, reducing the dog’s symptoms of itching, painful skin. They also wanted to assess if PCSO-524 could reduce the “rebound” of symptoms when oclacitinib dosages were reduced. They found that across the trial period of 42 days, the clinical condition in all groups improved, but in the PCSO-524 group, their condition appeared to improve more, when compared to the control group. Importantly, they also found that when oclacitinib dosages were reduced after 14 days, the risk of a rebound of uncomfortable symptoms was reduced in the PCSO-524 group when compared to the placebo.
The details
Why was this study done?
Canine atopic dermatitis is a complex skin condition caused by environmental allergens, with lots of factors feeding into it. It affects around 10% of all dogs and requires lifelong care, using both topical products, such as medicated shampoo or cream, and internal medication. Together these help to control the skin inflammation, reduce soreness and keep animals comfortable.
One of the most uncomfortable symptoms for your dog, and most distressing to watch as an owner, is pruritic behaviours, commonly known as itching.
Research has allowed us to understand the processes involved in this condition and has resulted in medication that targets the mechanisms causing pruritis (itching) and dermatitis (skin inflammation). This means vets are better able to manage this disease.
However, these medications have some side effects, and in this study researchers look at oclacitinib, an immunosuppressant drug that targets some of the cytokines involved in the processes that cause inflammation and itching. It is very effective and is a popular treatment.
Usually, Oclacitnib is recommended at a higher dose for 14 days before reducing the dose to half to maintain the dog’s skin health in the long term. However, there is a lot of evidence of atopic dermatitis rebounding when the dosage is dropped. This causes an increase in pruritogenic cytokines and fast sensitization of the skin, resulting in dogs feeling incredibly itchy, perhaps even worse than before.
Previous studies have shown that using polyunsaturated fatty acids (PUFAs) alongside antihistamines, corticosteroids, or ciclosporin may allow for better symptom control or a reduction in the dose of the drugs. This study aims to see if PCSO-524, a patented blend of marine lipid oils containing over 91 different fatty acids, could work in synergy with oclacitinib—particularly when reducing the dose of oclacitinib. PSCO-524 has been shown in previous research to demonstrate anti-inflammatory properties and is primarily used to support dogs and cats with osteoarthritis.
What did we expect to find?
Read our explanation of what makes a good study here first.
It was hypothesized that as PCSO has a great anti-inflammatory effect in osteoarthritis and has been shown to work in combination with other drugs, perhaps its effects could support the use of oclacitinib and even reduce the rebound effect when the dosage is dropped, reducing itching and improving the dogs’ quality of life.
Researchers are always looking for “statistically significant” results. This means that when the results are analyzed, you can be confident that those results didn’t just happen by chance.
Where does the study fit into the hierarchy of evidence?
Figure 1. Hierarchy of evidence
Not sure what the hierarchy of evidence is? Check out the clinical studies basics here.
This was a prospective, randomized, double-blinded, placebo-controlled clinical trial. It sits in the red zone of the hierarchy of evidence (Fig.1), which is generally considered to be a study of robust design, particularly useful for primary trials looking at treatment or prevention of conditions.
The study was double-blinded, which means neither the owner nor the veterinary team knew which owner and dog team had been given either the supplement (PCSO-524), or the placebo (sunflower oil).
The placebo part of the trial is particularly important as the “caregiver placebo effect” has been observed in other clinical trials. This is when the caregiver (whether that be an owner or a member of the veterinary team) perceives a difference in that pet because they know they have given them something new, even though when a computer analyses the results, no improvement is measured. Giving a placebo in place of the actual test substance means the researcher can remove any caregiver effect.
How was the study set up?
Seventeen client-owner pet dogs with Canine Atopic Dermatitis (cAD) were randomly assigned into two treatment groups. Dogs were either given oclacitinib combined with PCSO-524, or were assigned to the placebo group, receiving oclacitinib with sunflower oil.
If they were being given any treatment for cAD before the study, they had to stop them for at least two weeks before the study started. If they were already on long-acting glucocorticoids, they couldn’t be included in the study.
All dogs received oclacitinib (0.4-0.6mg/kg) twice a day for 14 days, then once a day until day 42.
The PCSO-524 capsules contain a proprietary mix of PCSO-524 (50mg), olive oil (100mg) and vitamin E (D-alpha-tocepherol: 0.225mg). The placebo capsules were designed to look the same as the PCSO-524 capsules, but they contained sunflower oil (139.5mg/capsule), water, and glycerin.
What was measured?
All dogs in the trial were seen by the same veterinary surgeon on day 0, day 14, day 28 and day 42. All dogs started taking oclacitinib plus their assigned treatment on day 0.
CADESI-04
Each dog was assessed by the veterinary surgeon, and its clinical state and pruritus levels were evaluated using the Canine Atopic Dermatitis Extent and Severity Index, 4th Iteration. This scale is used by vets to assess how bad a dog’s skin condition is. It looks at the lesions on the skin, how red and sore they are, and how itchy dogs are. This was assessed at days 0, 14, 28, and 42.
pVAS
There was also an owner assessed pruritus visual analogue scale done at each visit. This is an owners view of how bad their dog’s skin condition was. It is designed to assess how severe the dog’s itching and scratching behaviors are on a scale from 1 (normal dog) to 10 (extreme itching).
Trans Epidermal Water Loss
Trans epidermal water loss is a measure of how much water is being lost from the skin. It’s a normal process that happens throughout the day. However, if an animal suffers from atopic dermatitis, the rate of water loss is faster. It’s an indicator that the skin’s natural barrier function isn’t working correctly. So, if a dog in the trial has a reduced TEWL as the trial continues, it is an indicator that their skin’s barrier function, and potentially their skin health, is improving.
What were the results?
CADESI-04
Both groups were assessed on day 0, 14, 28, and 42. They were assessed by the same veterinary surgeon each time.
Across all groups the CADESI-04 scores decreased compared to day 0. As expected, the scores in the placebo group decreased until day 14, and then rose again after that, although not to as high as the original value. This demonstrates the commonly reported rebound effect when the oclacitinib dosage was reduced. The dogs skin condition worsened again when the dosage was reduced.
However, in the PCSO group there was no evidence of this. Both groups showed a significant improvement in CADESI-04 between day 0 and day 14. At day 28 and 42 the PCSO-524 group shows significant improvement when compared to the placebo group. It also showed a significant improvement over time when comparing subsequent days to day 0 results.
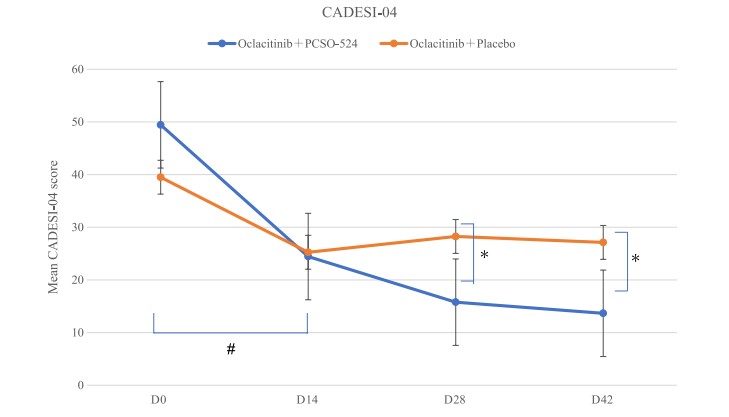
Figure 2. Effects of PCSO- 524 on Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI- 04) in the PCSO- 524 and placebo groups. The PCSO- 524 group demonstrated significant improvement compared to the control group at Day (D)28 and D42.
pVAS
The pVAS scores, which take into account the owner’s opinion on the severity of their dog’s condition, showed a similar story to the vet-assessed CADESI-04 results. Both the placebo group and the PCSO-524 groups showed a significant reduction in their scores between day 0 and 14. Once the dogs dropped their oclacitinib dose to half, at day 14, the placebo group rose a little and plateaued. However, the PCSO-524 group continued to see improvements in their scores, and there was a significant difference between the placebo group and the PCSO-524 group on days 28 and 42.
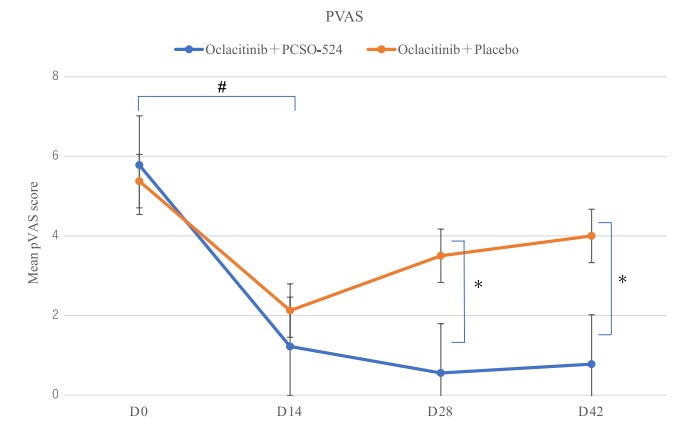
Figure 3. Changes in pruritus Visual Analog Scale (pVAS) after the oclacitinib combined with PCSO- 524 or control. Results show the significant decrease at Day (D)28 and D42 in the PCSO- 524 group.
TEWL
Despite the randomisation, the PCSO-524 group started at day 0 with a TEWL that was already significantly higher than the control group. However, by day 14 the PCSO-524 group’s TEWL had dropped lower than the placebo group. By day 28 and day 42 there was a significant improvement in TWEL in the PCSO-524 group when compared to day 0, but there was no significant improvement for the placebo group.
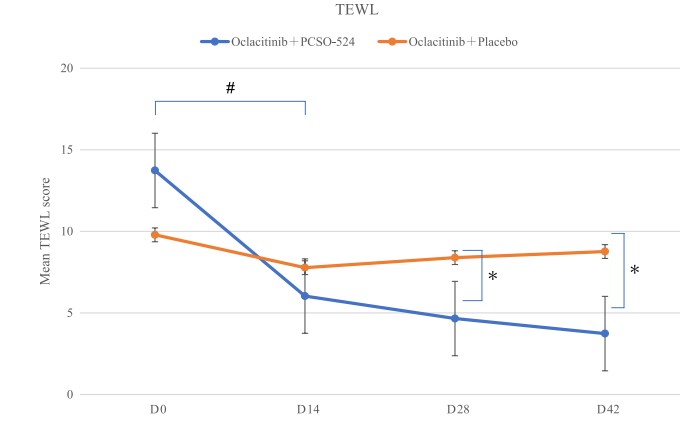
Figure 4. Changes in the trans epidermal water loss (TEWL) of the control and PCSO- 524 groups in the clinical trial. No significant effects were observed in the control group, while the PCSO- 524 group showed steady and significant improvement.
What do these results mean for my dog?
Atopic dermatitis is a horrible condition that severely impacts your dog’s quality of life. Studies have shown that oclacitinib treatment effectively reduces inflammation and pain and relieves itching. However, often when the dosage is reduced, the atopic dermatitis symptoms worsen again. In 33% of cases, dogs can’t be tapered down to the half dose after 14 days because their condition deteriorates so much that they are advised to remain on the higher dose. This in itself can be stressful for the dog and the owner, not to mention it’s more expensive as it requires more regular blood testing and comes with an increased risk of side effects.
This clinical trial demonstrated an improvement in the trial dogs’ clinical signs of atopic dermatitis and a reduction in pruritis, or how itchy the dogs feel, in the test group of oclacitinib and PCSO-524 when compared to the placebo group. The owner’s views of the dog’s skin condition in the test group were all within the normal range, compared to the placebo group, where only one dog achieved a normal result. This may be because of the anti-inflammatory properties of the functional ingredients in PCSO-524. These can modify the production of pro-inflammatory mediators and help reduce inflammation.
If your dog has atopic dermatitis, this combination of oclacitinib and PCSO-524 may be useful in controlling the symptoms of their skin condition and helping them to feel more comfortable.
Plus, when your dog needs to reduce their dosage of oclacitinib at 14 days, these results suggest that using PCSO-524 alongside oclacitinib may compensate for a potential rebound in the skin condition when the dosage is reduced. So, your dog’s condition may remain under control even when on a lower dose.
Although this is a small and short-term study, it is sufficient to draw conclusions and observe the effects. The researchers do however recommend that further testing is carried out with a larger group over a longer period of time, and where a controlled diet ensures the dogs are all receiving the same nutrition.
The positive outcome of this study gives vets and owners of dogs with atopic dermatitis confidence that they could support their dog’s skin health and reduce uncomfortable symptoms in the longer term by combining oclacitinib with PCSO-524, potentially reducing the risk of rebound itchiness.

