A double-blind, placebo-controlled clinical study comparing the effectiveness of green lipped mussel oil-based supplements Antinol+® (EAB-277®) and Antinol® (PCSO-524®), to a non-steroidal anti-inflammatory drug (NSAID) carprofen and glucosamine and chondroitin sulfate.
Summary
In this robust clinical trial, 75 dogs with osteoarthritis in their hips were given one of the following treatments for 6 weeks: Carprofen (Rimadyl®), EAB-277® (Antinol+®), PCSO-524® (Antinol®), glucosamine & chondroitin, or a placebo. Their ability to put weight on their affected limbs was assessed before treatment and every 2 weeks for a total of 6 weeks.
After 2 weeks, dogs on carprofen performed significantly better than the placebo group. By 4 and 6 weeks, Rimadyl®, Antinol+®, and Antinol® were all similarly able to help dogs bear weight on their affected limbs significantly better than before treatment, meaning the dogs were experiencing less pain in their arthritic limbs than before the trial. The glucosamine and chondroitin group were able to bear a little more weight from the starting point (base line) but sat below the other groups and the placebo.
Why was this study done?
Osteoarthritis (OA) in dogs is one of the most common conditions treated by veterinarians. Estimates suggest that 80% of dogs aged 7+ have OA, and it’s seen in 20% of dogs one year old and over. It is also a leading reason for premature euthanasia.
The most common drug used to treat pain in osteoarthritis cases is Rimadyl® (carprofen), a non-steroidal anti-inflammatory drug (NSAID). Although considered effective, NSAIDs may cause digestive problems and may not be suitable for dogs with certain other age-related conditions such as kidney disease.
The most commonly used nutraceutical supplement for osteoarthritis in dogs is a combination of glucosamine and chondroitin and, although very popular with dog owners, the scientific evidence for its effectiveness is limited.
For this study, Antinol+® which contains EAB-277®, was tested against a non-steroidal anti-inflammatory drug (Carprofen), Antinol® which contains PCSO-524®, another commonly used supplement (glucosamine and chondroitin sulfate), and a placebo.
What did we expect to find?
Read our explanation of what makes a good study here first.
It was predicted that the study would find that all treatments were more effective at treating osteoarthritis symptoms than the placebo and that carprofen, EAB-277® and PCSO-524® would be more effective than glucosamine and chondroitin.
Where does the study fit into the hierarchy of evidence?
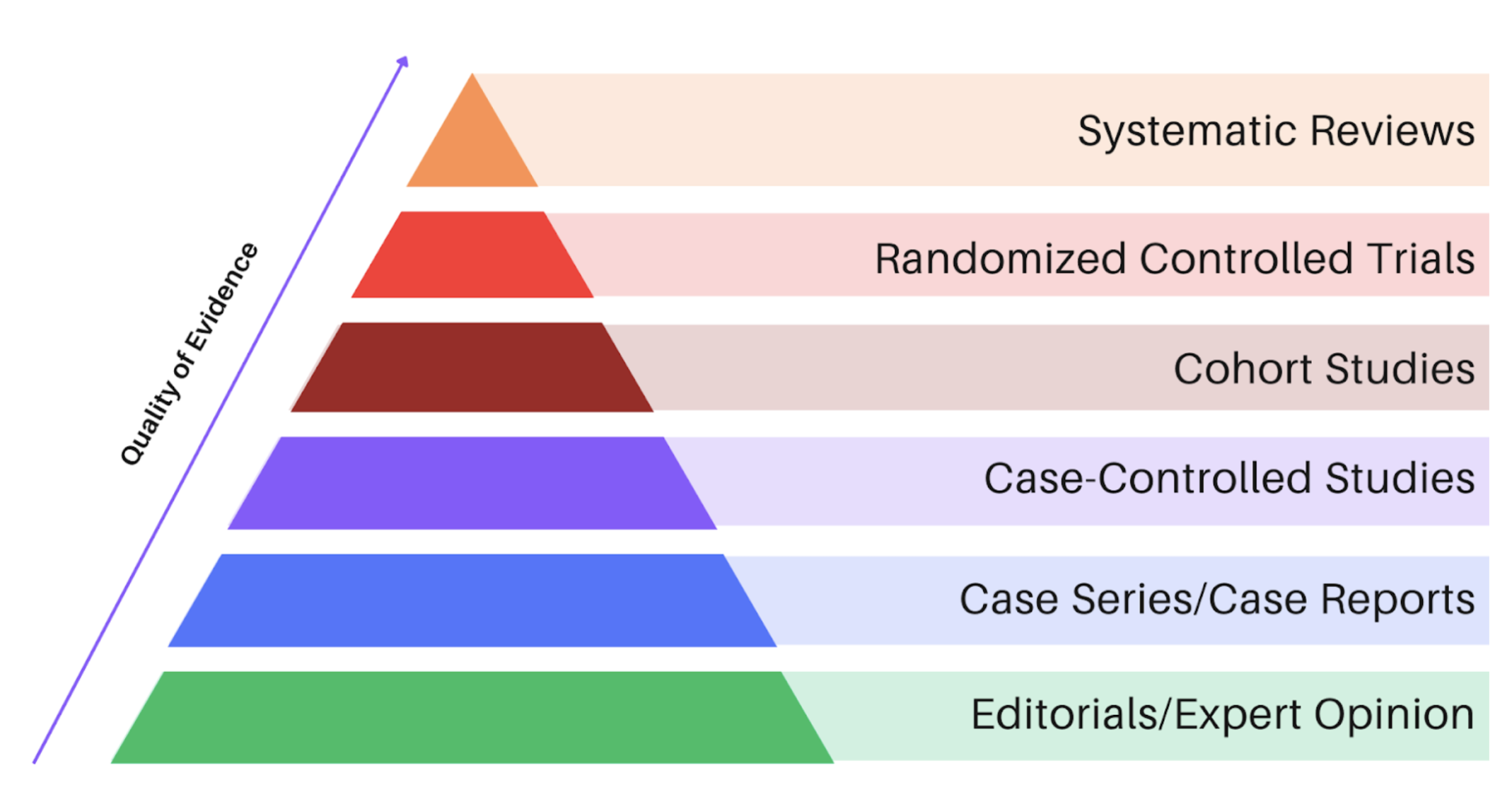
Figure 1. HierarchyHeirachy of evidence
Not sure what the hierarchy of evidence is? Check out the clinical studies basics here.
This was a prospective, block-randomized, double-blinded, placebo-controlled clinical trial. It sits in the red zone of the hierarchy of evidence (Fig.1), which is generally considered to be a study of robust design, particularly useful for primary trials looking at treatment or prevention of conditions.
The study was double-blinded, which means neither the owner nor the veterinary team knew which owner and dog team had been given which drug, supplement, or placebo.
It was also block-randomized, which randomly splits the trial dogs into equal-sized test groups, reducing the risk of bias which could skew clinical results.
The placebo part of the trial is particularly important as the “caregiver placebo effect” has been observed in other clinical trials. This is when the caregiver (whether that be an owner or a member of the veterinary team) perceives a difference in that pet because they know they have given them something new, even though when a computer analyses the results, no improvement is measured.
This is particularly relevant when considering their orthopaedic assessment score (OAS). Adding a placebo group means that when comparing the groups, the caregiver placebo effect can be taken into account. The placebo should be a substance that will have no clinical effect on the dog.
How was the study set up?
Seventy-five owned pet dogs with osteoarthritis (OA) in their hips were assigned randomly into five treatment groups. Peak vertical force (PVF) and subjective orthopaedic assessment scores (OAS) were evaluated before treatment (week 0) and at weeks 2, 4, and 6 during treatment.
The clinical trial was conducted over a six-week period.
What was measured?
Peak Vertical Force
Peak Vertical Force (PVF) helps us understand a dog’s weight-bearing ability. The dogs were walked across a sensor called a force plate, which measures how much body weight the dog puts on each leg. If a dog has pain in one or more limbs, he may put less weight on the affected limb(s).
The sensors mapped their gait (how the dog moves) which allowed us to compare their weight-bearing ability before, during, and after treatment. The ability to bear more weight after treatment is a good indicator that the dog is experiencing less pain or improved mobility.
Orthopaedic Assessment Score
An Orthopaedic Assessment Score (OAS) is a means of measuring the pet’s pain and mobility after clinical examination and may also consider the opinion of the owner.
In this case it was assessed at the veterinary hospital. This study considered the severity of each dog’s lameness, the range of motion of joints (how far the dog can move them), pain on touching the affected area, ability to bear weight, and overall clinical condition.
What were the results?
Week 2
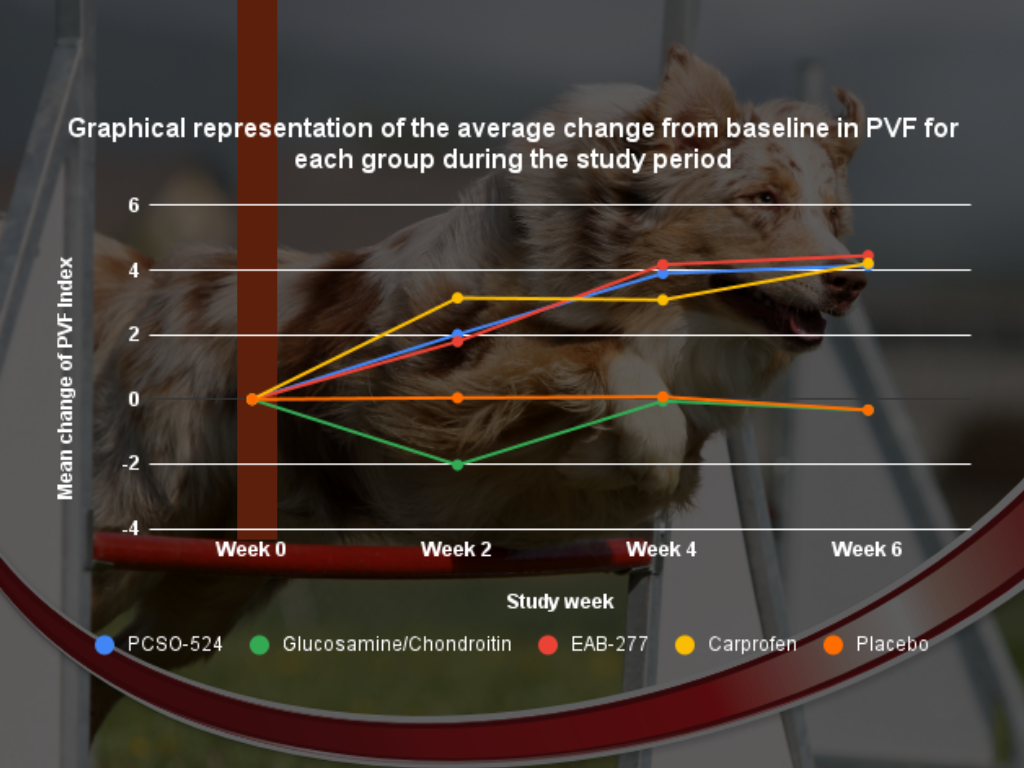
At week 2, the carprofen (NSAID) group showed a statistically significant increase in PVF, meaning the dogs were bearing more weight on their limbs than before the trial and more than the placebo group.
Antinol+® (EAB-277®) and Antinol® (PCSO-524®) also showed an increase in PVF, although not statistically different from week 0.
Glucosamine had a reduced, but not significant, decrease in PVF.
The placebo group remained unchanged.
Week 4
By week 4, the carprofen, Antinol+® (EAB-277®), and Antinol® (PCSO-524®) groups all had statistically significant increases in PVF.
Glucosamine and Chondroitin returned to their original value with no statistically significant difference in PVF than the placebo group.
Week 6
At the end of the study, carprofen, Antinol+® (EAB-277®), and Antinol® (PCSO-524®) all showed significant increases in PVF and there were no differences between these treatments.
Glucosamine and Chondroitin had improved PVF but lay between the other groups and the placebo group.
. 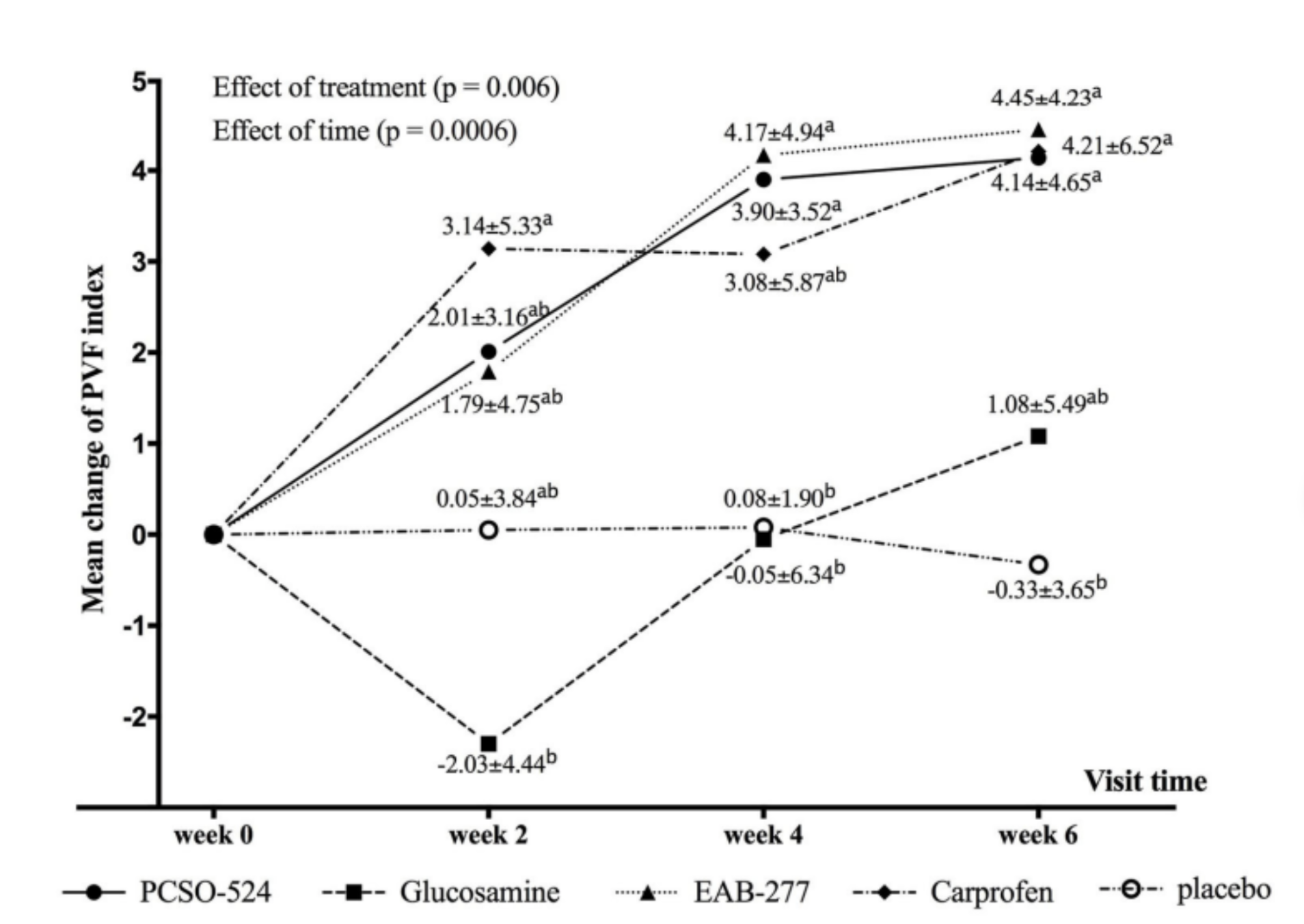
Figure 2. Graphical representation of the mean (± standard deviation) change from baseline in PVF for each group during the study period. Different superscripts (a, b) indicate significant differences between groups for change in PVF.
What do these results mean for my dog?
The NSAID carprofen, Antinol+® (EAB-277®) and Antinol® (PCSO-524®) all showed significant improvements in PVF after 4 and 6 weeks, meaning the dogs in the study were able to bear more weight on their legs than before treatment. This should mean that they experienced a reduction in the pain associated with osteoarthritis.
Currently, non-steroidal anti-inflammatory drugs (NSAIDs) are the first-line treatment used by vets in cases of osteoarthritis in dogs. This study suggests that carprofen is the fastest acting of the substances being tested, but that by weeks 4 and 6, Antinol+® (EAB-277®) and Antinol® (PCSO-524®) delivered similar results and, therefore, could be considered useful in cases where NSAIDs aren’t suitable or when the pet is in need of greater pain relief.
If you would like to read the full scientific study, you can download it here.


