Immune-mediated Hemolytic Anemia (IMHA) is a complicated, life-threatening disease causing dogs to become rapidly anemic, with widespread inflammation. Survival rates are low, but treatment development is still ongoing. Veterinarians usually start with corticosteroid treatments, which are effective but come with unpleasant side effects. This study aimed to investigate using EAB-277 (Antinol Rapid), a marine-lipid fatty acid extract, to improve the clinical outcomes for dogs with IMHA.
Eighteen dogs with IMHA were treated with Prednisolone (a corticosteroid) or a combination of Prednisolone and EAB-277. Their blood chemistry profiles were assessed, and survival rates and ability to reduce their prednisolone dosage were compared.
The study showed that blood profiles were improved with EAB-277 supplementation, resulting in a higher survival rate, and dogs could reduce their prednisolone dosage earlier. This suggested that EAB-277 is a promising treatment option for dogs with IMHA and may improve their overall condition and quality of life, in conjunction with prednisolone.
Why was this study done?
Immune-mediated hemolytic anemia is a common autoimmune disorder in dogs. The disease is complicated and life-threatening, with mortality rates varying from 30-70% and a 20% chance of relapse within the first year.
IMHA is an autoimmune disease, meaning that instead of working to protect the dog, its immune system attacks itself. In this case, it destroys its own red blood cells, resulting in anemia and poor oxygen delivery to organs. The disease also causes an increase in pro-inflammatory cytokines, which cause inflammation and damage throughout the body.
IMHA is a difficult disease to treat, and work is still ongoing to understand it and develop treatments. Currently, the most common treatment veterinarians use is corticosteroids, which suppress the immune system, giving it time to recover. Dogs are then slowly tapered off of the corticosteroids because long-term usage is not recommended.
Marine lipid extract EAB-277 contains a unique combination of polyunsaturated fatty acids (PUFAs) that have previously been shown to have a potent anti-inflammatory action. They are being used to support the treatment of various inflammatory conditions in dogs, including osteoarthritis and degenerative spinal diseases.
EBA-277 has the potential to support dogs suffering from IMHA, so researchers wanted to compare treatment outcomes for IMHA dogs on corticosteroid treatment, with and without EAB-277 supplements.
What did we expect to find?
Read our explanation of what makes a good study here first.
Supplementation with EAB-277 was predicted to reduce the amount of corticosteroid (in this case, prednisolone) needed, improve the dog’s health, and increase the survival rate compared to standard therapy.
Where does the study fit into the hierarchy of evidence?
Figure 1. Hierarchy of evidence
Not sure what the hierarchy of evidence is? Check out the clinical studies basics here.
This was a prospective, randomized, open-label, controlled clinical trial. It sits in the red zone of the hierarchy of evidence (Fig.1), which is generally considered to be a study of robust design, particularly useful for primary trials looking at treatment or prevention of conditions.
The study was an open-label trial, which means that both the dog owners and the researchers knew which treatment the dogs were given. This does mean the study has the potential for bias in the results, but it is an accepted study model for this kind of research.
It was also a randomized study, which means that trial dogs were randomly assigned to either the test or control group. This helps to limit skew in the results, although the numbers of dogs were not equal in the two groups.
It must be remembered that this is a preliminary study, which means it is testing the water and looking for positive results that can confirm if it is worth pursuing this line of research further. These studies are useful when there is little evidence to support the theory in the scientific literature. They set the scene for future research, which is why the testing protocols are not as strong as we expect in a full-scale clinical trial.
How was the study set up?
Eighteen owned pet dogs diagnosed with IMHA were randomly assigned to either the control group (prednisolone treatment) or the test group (prednisolone + EAB-277). The prednisolone treatment was given at the immunosuppressant dose and tapered following a standard tapering procedure. EAB-277 was given at 100mg/10kg bw for 14 days, followed by 50mg/10kg bw for 14 days, as recommended by the manufacturer.
Researchers assessed each dog to verify its diagnosis and ensure there were no underlying causes of its anemia. Dogs couldn’t be included if they had ever received blood products or had previously had immunosuppressants for more than 48 hours.
Animals were observed at 0, 7, 14 ,21 and 28 days for any clinical changes and survival rates, and blood was taken and analysed at these times.
What was measured?
Initially, researchers analysed the dog’s blood for the presence of spherocytosis and agglutination, which are both indicators of IMHA. None of these were statistically different between the two test groups at the start, suggesting that the severity of IMHA was similar between the two groups.
- Clinical pathology
Various blood parameters were tested at five time points, including red blood cell count, hematocrit levels, haemoglobin levels, and platelet counts. These give an indication of how well the dog is managing the disease and whether it is worsening or improving. White blood cell and neutrophil levels were also measured.
General health was assessed using the blood test results to examine the impacts on various organs, such as the kidneys and liver.
- Survival Outcomes
The survival rates of dogs were recorded and compared between groups. The study looked at the overall survival rate within each group and the survival time post-therapeutic treatment.
- Tapering of Prednisolone
The dose of prednisolone given to the dogs began to be reduced as they improved, and HCT had been stable at greater than 30% for two weeks. The dosage was reduced by 25% each time. The number of dogs who were able to taper their prednisolone and the rate at which they tapered were compared between groups.
What were the results?
- Clinical Pathology
There was no difference in red blood cell count, Hematocrit, Hemoglobin, or platelet count between the two groups; however, all erythrocyte parameters were statistically increased at 28 post-therapeutic days in the supplement group, and the platelet count was also significantly increased at days 14 and 21 when compared to the baseline.
Although not significantly different, it should also be noted that the WBC and neutrophil count were lower in the supplement group 21 days post-treatment. Serum Alkaline Phosphatase was also higher in the control group, indicating potential liver damage.
- Survival Outcomes
The mortality rate was higher in the control group than the supplement group, and the survival rate declined to 16.7 at 28 days post-therapy, whereas in the supplement group, the survival rate was much higher, at 66.7 throughout the study.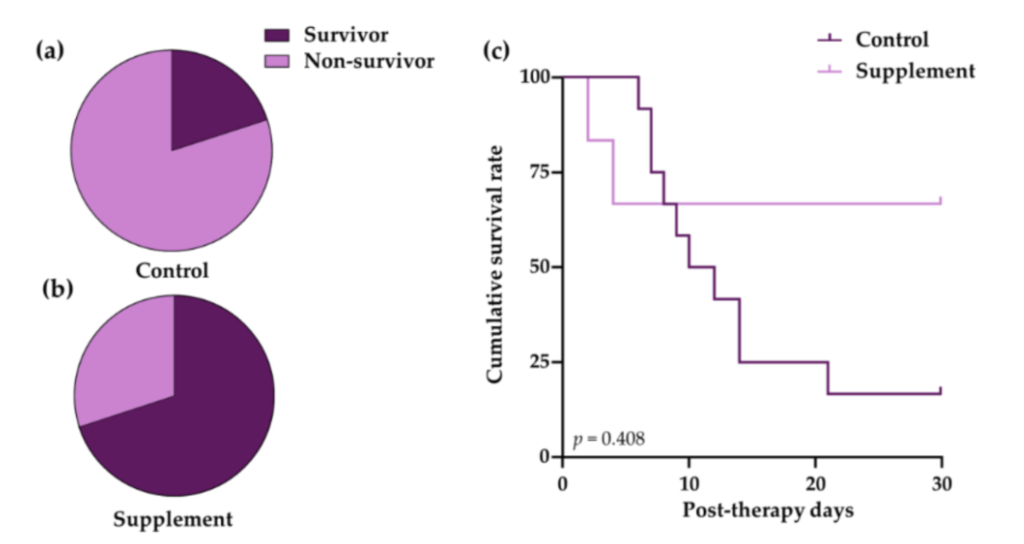
Figure 2. The mortality and survival analysis of IMHA dogs treated with prednisolone alone (control group) and prednisolone combined with EAB-277 (supplement group). Pie chart to show percentage mortality in (a) control group and (b) supplement group at 28 days after treatment. Fisher’s exact test was used for statistical analysis; (c) Kaplan–Meier curve showing difference in survival between control group (light purple line) and supplement group (dark purple line). The log-rank test was used for statistical analysis.
- Prednisolone Tapering
The number of dogs able to taper their prednisolone dose was higher in the supplement group. EAB-277-supplemented dogs had 4.20 times the tapering rate compared to the control group.
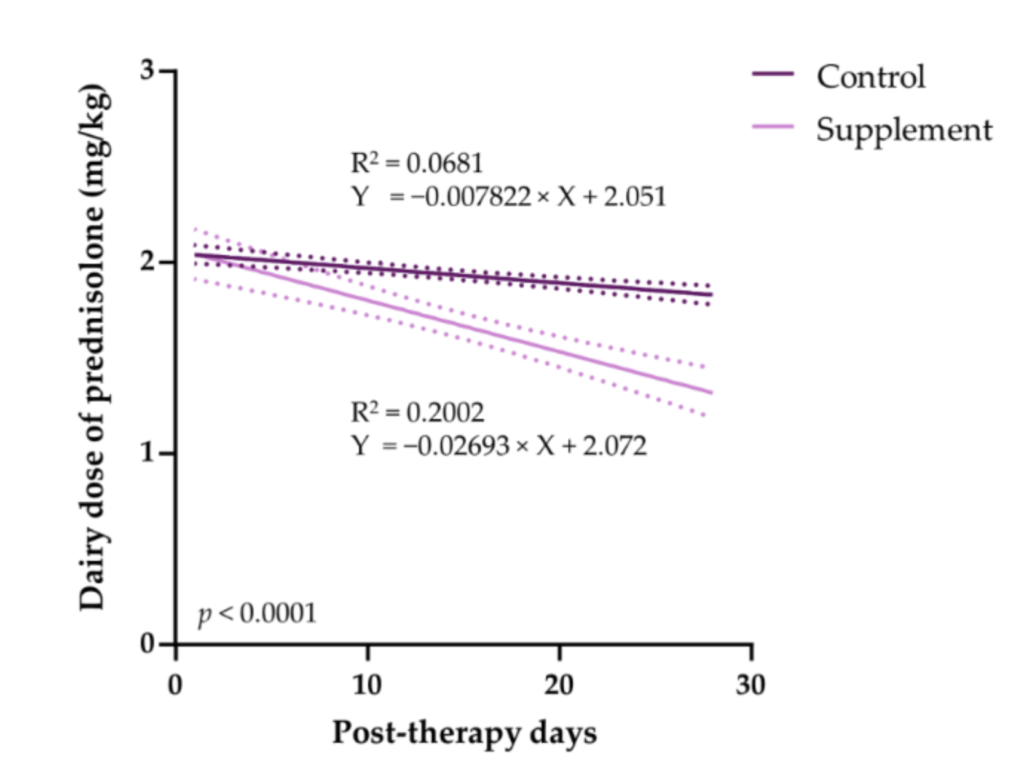
Figure 3. Comparison of the daily dose of prednisolone in IMHA dogs treated with prednisonole alone (control group) and prednisolone combined with EAB-277 (supplement group) for 28 days.
What do these results mean for my dog?
Past studies have demonstrated the anti-inflammatory properties of EAB-277, and this study backs this up. It also suggests that using EAB-277 with standard prednisolone therapy can support your dog’s IMHA recovery.
EAB-277 supplementation resulted in a better survival rate for dogs compared to standard therapy. Although this wasn’t statistically significant, there was also a decrease in the attack on red blood cells in the supplement group, suggesting the dogs’ conditions were improving. The supplement group also showed a decrease in inflammatory response with reductions in WBC and neutrophil count.
Dogs could also reduce their prednisolone more quickly when supplemented with EAB-277. Long-term prednisolone usage comes with many potentially unpleasant side effects, which are uncomfortable for the dog and often mean that owners aren’t keen to continue therapy. Using EAB-277 alongside standard therapy suggests that prednisolone tapering could be quicker, improving their dog’s quality of life.
IMHA is a horrible, life-threatening disease which can take hold rapidly and is distressing for both your dog and you. Although more research needs to be done, this preliminary study is positive. Previously, safety studies show that long-term use of these supplements is safe, so if your dog is struggling with IMHA, there is no harm in trying it alongside standard therapy as it may support their treatment and improve their quality of life.
If you would like to read the full scientific study, you can download it here.


